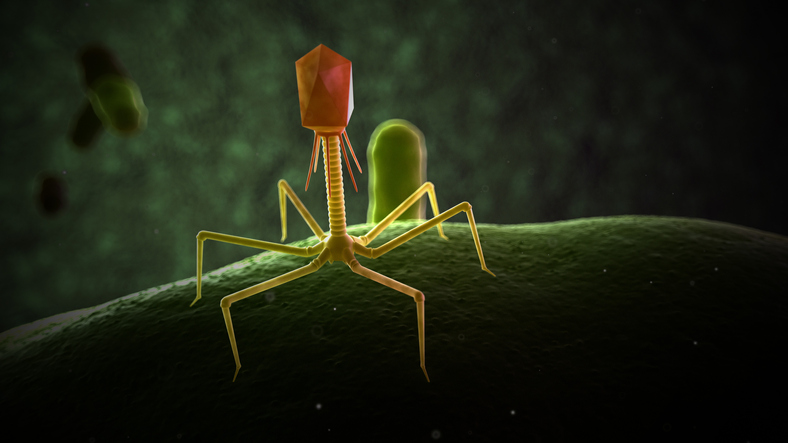
Bacteriophages, or phages for short, are abundant and ubiquitous viruses. They are part of nature and are everywhere, in the air, oceans, soil, and in every living being. In humans, they are especially abundant in our guts. If the number of bacteria in our bowels can reach trillions, it is estimated that there are 4–10 phages for every single bacterium.
These viruses were discovered in the early 20th century, independently, by two researchers, Frederick Twort and Félix d’Hérelle. It was the French, d’Hérelle, who first proposed the use of phages as antimicrobials and later made great strides in promoting them for the treatment of bacterial infections. (Figure 1).
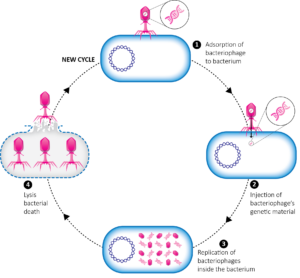
In the pre-antibiotic era, the idea was embraced by hospitals and pharmaceutical companies, such as Eli Lilly. Published scientific reviews indicate that the use of phages spread throughout the world, Brazil included1. In 1924, the Oswald Cruz Institute in Rio de Janeiro started a phage program to treat dysentery. For his achievements, d’Hérelle was nominated eight times for the Nobel prize, but was never awarded one.
The introduction of antibiotics in the 1940s, a more efficacious, cheaper, and less complex alternative to treat bacterial infections, changed this picture. Although research on phages continued in the Soviet Union and Poland, it was mostly abandoned by the rest of the world. D’Hérelle left the Pasteur Institute in Paris for the George Eliava Institute, founded in 1923, in Georgia. George Eliava, the founder who gave his name to the institute, was killed during the Stalin regime.
Nevertheless, the institution bearing his name persisted and is now an active consortium which holds a large collection of phages. The consortium is composed of a diagnostic center, pharmacy, foundation, research institute, and a company that commercializes bacteriophage-based products for human health, veterinary applications, and the environment.
Business opportunity for Latin America
Antibiotic resistance is a growing and serious problem. Last year, The Lancet published the following statistics: in 2019, there were 1.27 million deaths associated with anti-microbial resistance. For the sake of comparison, more people died as a result of drug-resistant bacterial infection in 2019 than of breast cancer.
The Global Antibiotic Research & Development Partnership (GARDP), a joint initiative of the WHO and the Drugs for Neglected Diseases (DNDi), is dedicated to the development of solutions for antibiotic resistance. It estimates that, by 2050, 10 million people can die every year due to antibiotic resistance. This is one of the top ten global health threats and it cannot depend on the current pharmaceutical pipeline, as it is not sufficient to tackle the problem.
This worrisome scenario brought back an interest in the use of phages as antimicrobials. Money for research has increased in the world’s biotechnology leader, the U.S. The number of articles in the medical literature tripled between 2016 and 2022.
The University of California in San Diego created a Center for Innovative Phage Applications and Therapeutics in 2018. It collaborates with the Center for Phage Technology, established eight years earlier at Texas A&M University. The number of articles in the medical literature increased greatly since 2014, and so have clinical trials. There were 39 in 2014, now there are 81.
Of these 81 trials, four types of sponsor companies were identified: 1) companies dedicated to optimizing methods to isolate, characterize, manufacture and develop phage-based formulations; 2) companies doing the same procedures as the previous group but using genetic engineering and gene editing technologies to create novel bacteriophages; 3) companies that are, in addition to making phage formulations, developing technology platforms based on individual molecules derived from phages; and 4) diagnostic companies that use the specificity of phage:bacterium recognition to develop diagnostic tests.
Diabetic foot ulcers, urinary tract infections, inflammatory bowel disease, cystic fibrosis, atopic dermatitis, skin care, colorectal cancer and infected prosthetics are recurrent therapeutic focuses of these clinical trials.
Phage-based medical treatments are yet to be approved by the regulatory agencies, FDA, EMA, or ANVISA, but clinical trials and compassionate use programs indicate that they are safe. There are plenty of published case reports showing that patients had their lives saved by using phage therapy. These were cases in which infections were not receding, despite all available treatments.
Companies and research laboratories have partnered with hospitals and pharmaceutical companies to test their proprietary technologies. BiomX, a spinoff of the Weizmann Institute, has partnerships with Janssen, Boehringer Ingelheim, and Maruho. Adaptive Phage and PrecisioBiotix have partnered with the Mayo Clinic. In 2022, Johnson & Johnson participated in a $35 million financing round in North Carolina-based Locus Biosciences from North Carolina.
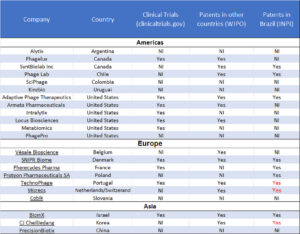
Many companies around the world, betting on the business opportunity, are depositing their patents in Brazil via the Patent Cooperation Treaty to protect their technologies, e.g., the American Adaptive Phage Therapeutics, the Israeli BiomX, the French Pherecydes, the Danish SNIPR Biome, the Dutch/Swiss Micreos and the Portuguese Technophage. The last two have patents granted by the INPI (the BRPTO, Instituto Nacional de Propriedade Industrial) (Table 1).
Phage-based formulations and products, despite not being approved for human care, are already being sold and used by the agribusiness and the food industry. In fact, the biggest number of patent deposits in Brazil are in this area. Few would disagree that phage biotechnology can be an interesting business opportunity. It has, in fact, fomented the creation of biotechnology companies in Latin America. Argentina, Chile, Colombia, and Uruguay have initiatives entirely dedicated to phages. PhageLab, from Chile, with operations also in Brazil and Spain, is the only biotechnology company in Kaszek’s portfolio of 107 investments in Latin America. The $30 million investment PhageLab received from Humboldt and Kaszek5 gives the company a unique position in the region.
Phages not only have the potential to help with the problem of antibiotic excessive use but can also be part of the urgently needed solution to fight the problem of antibiotic-resistant super bugs. However, a note of caution is called for. The overuse and misuse of antibiotics is pointed as one of the culprits of the existing threat of bacterial resistance. If the same mistakes are repeated, super bugs that are not only resistant to antibiotics but also to phages will be selected. And then what?
Denise Golgher, PhD, received her PhD from Johns Hopkins University and completed a post-doctoral fellowship at the University of Oxford. She is a life sciences consultant at Licks Attorneys in Brazil. Roberto Rodrigues, LLM, is an editor of the Kluwer Patent Blog and a partner at Licks Attorneys.