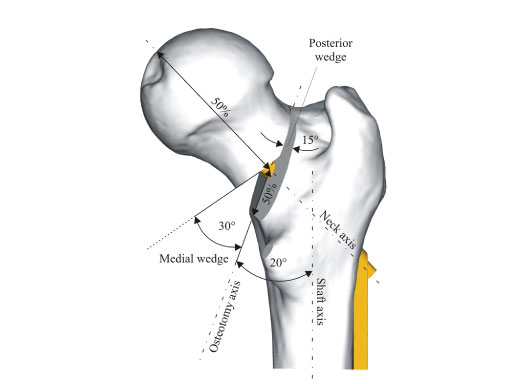
Dynamic Hip Screw (DHS) Blade
Christoph Sommer
The dynamic hip screw (DHS) blade is indicated for pertrochanteric fractures of type 31-A1 and 31-A2, intertrochanteric fractures of type 31-A3 (in combination with the trochanteric stabilizing plate), and femoral neck fractures 31-B. It is especially appropriate for use in elderly people with osteopenic/porotic bone.
Due to the shape of the blade and the locking mechanism there is better rotational stability of the femoral head neck fragment in comparison to the DHS screw which reduces the risk of cut-out, delayed union and varus angulation in unstable trochanteric fractures. The blade allows for compaction of the bone which leads to better anchorage of the implant in the femoral head, especially beneficial in osteoporotic bone. In a cadaver model performed at the AO Research Institute Davos, the superior performance of the DHS blade regarding cut-out resistance compared to the DHS screw could be shown (see below).
The surgical technique of the DHS blade is very similar to the existing DHS. The only difference is that the DHS blade is hammered into position compared to the screw which is turned into position. The rotational locking of the DHS blade takes place after the alignment of the DHS plate onto the femoral shaft to enable a rigid construct. The locking takes place with a special screwdriver and a 1.5 Nm torque-limiting attachment. No additional antirotational screw cranial to the DHS blade is necessary (contrary to the original DHS screw).
The DHS blade is fully compatible with the existing DHS plates. It is available from 65 mm to 145 mm in increments of 5 mm in both stainless steel and titanium. It is only available sterile.
Treatment of Unstable Femoral Neck Fractures
Is the DHS Blade a Superior Alternative to the Dynamic Hip Screw?
Objective
The dynamic hip screw (DHS) is a well established implant for treatment of trochanteric and femoral neck fractures. However, cut-out of the screw as a postoperative complication occurs in up to 16% of all clinical cases. The DHS blade has been developed as an alternative to the screw to reduce the cut-out rate. This in vitro study compares the DHS blade to the standard DHS with regard to cut-out resistance of the implant in an unstable femoral neck fracture model.
Materials/methods
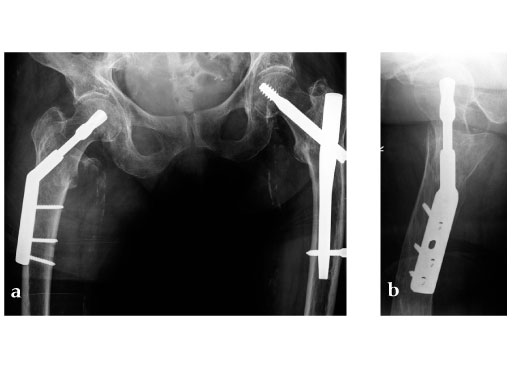
Ten pairs of human cadaveric femora were used. Left and right bones of every femoral pair were randomly instrumented with either a DHS blade or a DHS using 4-hole side plates. Tip-apex distance was standardized to 10 mm. After implantation an unstable type 31-B2 fracture (Fig 1) was created using a custom-made saw guide. Cyclic loading was performed to the femoral head applying load trajectories as measured in vivo in total hip replacement (THR) patients (Bergmann et al., 2001). The passive function of the iliotibial band was simulated by a cable. Starting at 1500 N the peak load was increased by 500 N every 5000 cycles until cut-out or complete failure of the construct. X-rays were taken at 5000 cycle intervals. A survival analysis was performed based on the numbers of cycles until cut-out, defined as the first visible implant migration as determined from x-rays.
Results
With the applied loading regime a total of 100% cut-outs occurred in the DHS group compared to 50% in the DHS blade group. The survival probability in terms of cut-out resistance was significantly higher for the DHS blade (Fig 2) (P=.023).
Conclusion
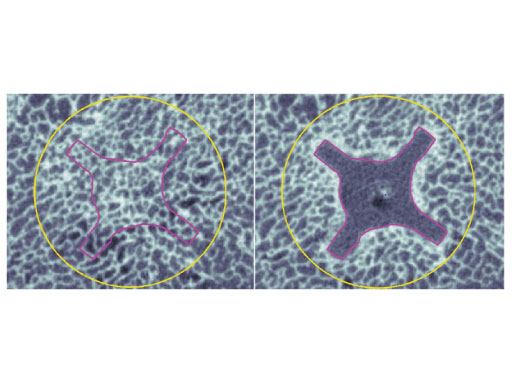
Based on the data available, we conclude that the DHS blade performs superior compared to the DHS in terms of cut-out resistance under cyclic loading. This might be due to cancellous bone compaction around the helical blade during implant insertion (Fig 3), which should be investigated in further studies. This in vitro study supports the usage of the DHS blade in order to reduce the cut-out rate in treatment of unstable femoral neck fractures.
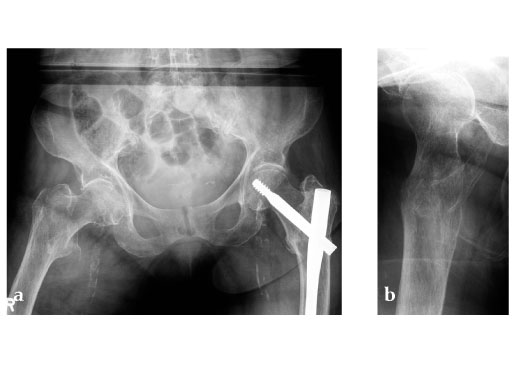
Case provided by Christoph Sommer, Chur, Switzerland
Müller AO Classification type 31-A2 fracture in an 83 year-old female with severe osteoporosis.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.