Abstract
The conversion of CO2 into fuels and valuable chemicals presents a viable path toward carbon neutrality. The aim of this study is to investigate the potential of metal-doped graphene catalysts in the reduction of CO2 to C1 products. 20 typical M-graphene (M = metal) catalysts were established based on DFT calculations. Six candidate catalysts, i.e., V-, Cr-, Mn-, Ni-, Mo-, and Ta-graphene catalysts, were selected by combining the hydrogen dissociation ability and the energy band gap of the catalysts. Subsequently, the adsorption characteristics and hydrogenation reactions of CO2 over the six candidates were explored. CO2 tends to adsorb at the M site through vertical adsorption and carbon–oxygen co-adsorption. V- and Cr-graphene catalysts promote the production of intermediate COOH, whereas Mn-, Ni-, Mo-, and Ta-doped surfaces are more favorable for HCOO formation. Concerning the hydrogenation to CO and HCOOH, V-, Cr-, Ni- and Mo-graphene catalysts preferentially yield CO from COOH, whereas Ta-doped graphene favors the formation of HCOOH. In total, the competitive hydrogenation of CO2 reveals the selectivity of the C1 products. Cr- and Ni-graphene favor the production of HCOOH and CH3OH, whereas V-, Mn-, Mo-, and Ta-graphene primarily yield CH3OH.
Graphical Abstract

Similar content being viewed by others
1 Introduction
The widespread reliance on fossil fuels has led to the emission of large quantities of CO2, intensifying the greenhouse effect and seriously affecting the global climate. The capture and utilization of CO2 play a pivotal role in fostering a sustainable model for economic development [1, 2]. Catalytic hydrogenation represents a crucial method for transforming CO2 into C1 molecules such as carbon monoxide (CO), formic acid (HCOOH), methanol (CH3OH), and methane (CH4) [3, 4]. Overcoming the inherent stability of the CO2 molecule poses a significant hurdle in its conversion, and thus, the innovation of effective catalysts is imperative for efficient CO2 hydrogenation [5, 6]. Graphene is a compact stack of carbon atoms with a honeycomb planar sp2 hybridized structure, which was discovered by Geim and Novoselov [7, 8]. Owing to its remarkable thermal and mechanical stability, alongside its high availability and flexibility, graphene is emerging as a promising candidate for hydrogenation catalysis [9]. The high surface-to-volume ratio of graphene makes it the ideal support for various catalytic reactions. However, the strong sp2 binding between carbon atoms in the pristine graphene leads to chemical inertness [10, 11].
Atomic surface modifications of graphene, such as doping with noble and transition metals, have been found to significantly enhance its catalytic performance and gas adsorption capabilities [12]. These modifications have shown promising results in the catalytic reduction of CO2 to C1 compounds. For instance, Au-doped graphene has been reported to lead to higher selectivity for HCOOH than other C1 compounds in CO2 conversion [13]. Similarly, transition metal Sn [14], Ti [15], and Co [16] doped graphene catalysts demonstrated a pronounced selectivity for HCOOH formation. Pd [17], Ag [18], and Fe [19] doped graphene catalysts predominantly yielded methane (CH4) as the hydrogenation product. Interestingly, the selectivity for CO2 hydrogenation can vary even when graphene is doped with the same metal. For example, San et al. [20] reported that CH4 was the main product in the CO2 catalytic conversion on Cu-loaded graphene. This differed from the findings of Ma et al. [21], which indicated a tendency to produce CO. Moreoverr, Wang et al. [22] found that the production of HCOOH in In-doped graphene, whereas Shi et al. [23] observed CH3OH production under similar conditions.
Generally, the C1 products are competitively generated by involving different intermediates in the CO2 hydrogenation process, and it is essential to get a deep insight into the formation and evolution of these intermediates over different catalysts to control the selectivity of C1 products [24]. Taking HCOOH for example, Yodsin et al. [25] identified HCOO as the key intermediate in the generation of HCOOH on Pt-doped graphene. Conversely, Esrafili et al. [15] discovered that the key intermediate was COOH on Ti-doped graphene for HCOOH formation, and COOH could lead to the formation of both HCOOH and CO via the hydrogenation at the C-site or the O-site, respectively. Moreover, CO and HCOOH could also be further hydrogenated into CH3OH or CH4. Based on density functional theory (DFT) calculations, H2COH has been recognized as a vital intermediate generated from the further hydrogenation of CO and HCOOH, and it could turn into CH3OH through competing pathways [26]. Szkaradek et al. [27] recognized the pathway for CH3OH generation on Ru-doped graphene by DFT calculation, which is CO2 → COOH → CO → COH → H2CO → H3CO → CH3OH. In total, the formation mechanism and competitiveness of C1 products over different metal-doped graphene by catalytic hydrogenation of CO2 still need further investigation.
This study focuses on identifying promising metal-doped graphene catalysts that can selectively produce specific C1 products, while also unraveling the competitive mechanisms of different hydrogenation products. 20 typical M-graphene (M = noble metal or transition metal) catalysts were built based on DFT calculations. The screening process was initially performed by comparing the hydrogen dissociation ability and the energy band gap of the catalysts. Based on the initial assessments, the electronic structures of the catalyst surface and the adsorption configurations of CO2 were analyzed. Subsequent investigations delved into the hydrogenation reactions leading to the formation of C1 products from adsorbed CO2. Particularly, the competitiveness of these C1 products and the catalytic mechanism of the promising catalysts were carefully discussed.
2 Methods
The DFT method was conducted using the DMol3 software package [28]. The Perdew-Burke-Ernzerhof (PBE) functional was in conjunction with a double numerical basis (DNP) using a 2 × 2 × 1 k-point mesh (the convergence test result is illustrated in Fig. S1). The influence of Hubbard (U) parameters and van der Waals forces on CO2 hydrogenation over the surfaces of M-graphene were tested. The results (Tables S1 and S2) indicate that DFT + U and van der Waals forces have no significant effect on the distribution of reaction energy. Therefore, + U and van der Waals forces are not included in our calculations. Details regarding the specific computational parameters and calculation formulas of adsorption energy (Eads) as well as energy barrier (Ebarrier) are shown in the Supplementary information. Electronic structure analyses were also conducted to reveal the role of catalysts in CO2 adsorption and hydrogenation, with detailed computing methods shown in the Supplementary information.
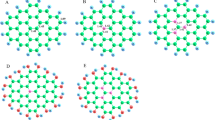
The pristine graphene was modeled as a monolayer flake of finite size with 50 carbon atoms, and its optimized configurations are shown in Fig. 1(a). For the M-graphene, the central C atom in this structure was substituted with various noble (Ru, Rh, Pd, Ag, In, Ir, Pt, and Au) and transition metals (Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Mo, In, Sn, and Ta), as demonstrated in Fig. 1(b).
3 Results and discussion
3.1 Catalyst screening
The promising metal-doped graphene catalysts are expected to exhibit both thermal stability and high catalytic activity. The thermal stability of all the M-graphenes built herein is affirmed according to their doping energies (Ed), which are in the range of 984.57 − 1871.75 kJ/mol (Table S3). To evaluate their catalytic activity, two key parameters are considered, which are the dissociation energy barrier (EDiss_barrier) of H2 on the catalyst surface and the energy band gap (Egap) of the catalyst. These criteria are crucial for screening promising candidates for CO2 catalytic hydrogenation.
Firstly, the adsorption and dissociation of H2 were examined. As indicated in Table S4, the adsorption energies of H2 molecules on the M-graphene surface range from -250.29 to -782.82 kJ/mol, which belongs to chemisorption. It indicates that H2 can be easily adsorbed on all these M-graphene surfaces and then undergo dissociation. The catalysts with lower EDiss_barrier are more favorable for the dissociation of H2 to generate active hydrogen. As shown in Table S4, the corresponding EDiss_barrier has a wide range from 56.26 to 304.77 kJ/mol, and the incremental order of energy barriers for the catalysts is Mn-, Ta-, Mo-, Cr-, Ru-, Pt-, Ni-, V-, Cu-, Co-, Au-, Pd-, Rh-, Zn-, Fe-, Ti-, Ir-, In-, Sn-, and Ag-graphene.
Secondly, the Egap values of M-graphene catalysts are calculated (Table S5). The electron transfer efficiency of the catalyst is influenced by its Egap. A small Egap promotes electron migration from the valence band to the conduction band, enhancing surface electron mobility. This contributes to the enhancement of the adsorption and activation of reactants and improves the surface activity of the catalyst. Moreover, the oxidation–reduction capability of catalysts correlates closely with their Egap. A narrow gap typically signifies a strong oxidation–reduction ability, enabling more effective participation in electron transfer reactions [29, 30]. Compared with graphene, Egap values of M-graphene catalysts are reduced, indicating that the introduction of metal is conducive to the electron transfer of graphene, and improves the ability of the catalyst to adsorb and activate molecules [31]. For effective CO2 hydrogenation, ideal candidates should possess low Egap values, and the decreasing order of Egap for the catalysts is Sn-, Pt-, In-, Ir-, Co-, Rh-, Ru-, Pd-, Cu-, Fe-, Ag-, Ti-, Ni-, V-, Ta-, Mn-, Zn-, Cr-, Mo-, and Au-graphene.
Finally, the correlation diagram between EDiss_barrier and Egap of M-graphene catalysts is presented in Fig. 2. The top 10 candidates with low EDiss_barrier and the top 10 candidates with low Egap are screened based on Tables S4 and S5. respectively, and six candidate catalysts are obtained with lower values of both EDiss_barrier and Egap (in the red frame in Fig. 2), i.e., V-, Cr-, Mn-, Ni-, Mo-, and Ta-graphene. According to the electron density difference (EDD) in Fig. S2, there are significant changes in the charge distribution for the six M-graphene structures in comparison to the pure graphene surface. It indicates that the doping of these metals significantly enhances the activity of the graphene catalyst surface, corroborating the screening results based on EDiss_barrier and Egap. The six candidates are employed to further investigate the catalytic hydrogenation potential of CO2. The PDOS maps of the six candidate catalysts in Fig. S3 show significant hybridization between the d-orbitals of the metal atoms and the p-orbital of the C atoms, indicating the charge transfer between the metal and C on the catalyst surface. Additionally, the d-band centers of the six M-graphenes have positive displacements compared to graphene (Fig. S4), and thus the introduction of the six metals is beneficial for attracting and activating molecules.
3.2 Adsorption mechanism of CO2 on the catalyst surface
There are two types of CO2 adsorption models, i.e., linear CO2 adsorption (parallel, vertical) and bent CO2 adsorption (dioxygen, carbon, carbon–oxygen) [32], as illustrated in Fig. S5. The adsorption configurations of CO2 on the surface of M-graphene catalysts (M = V, Cr, Mn, Ni, Mo, Ta) were constructed using the above two types of adsorption models. Two stable adsorption configurations were obtained, namely linear CO2 vertical adsorption and bent CO2 carbon–oxygen co-adsorption, as displayed in Fig. 3. The adsorption energies of linear CO2 vertical adsorption are -490.72 kJ/mol (V-graphene), -412.14 kJ/mol (Cr-graphene), -319.22 kJ/mol (Mn-graphene), -293.94 kJ/mol (Ni-graphene), -640.50 kJ/mol (Mo-graphene), and -802.18 kJ/mol (Ta-graphene). The adsorption energies of carbon dioxide carbon–oxygen CO2 adsorption are close to that of CO2 vertical linear adsorption, which belongs to chemisorptions. Among them, CO2 adsorbed on Mo-graphene is the most stable (Eads = -844.39 kJ/mol) and the lowest adsorption energy is obtained on Ni- graphene (Eads = -287.72 kJ/mol). The above two adsorption types are based on the Lewis base sites provided by metals. The adsorption energies of bent CO2 carbon–oxygen co-adsorption are higher than those of linear CO2 vertical adsorption, except over Ni-graphene. Meanwhile, Fig. 3 features the electron density difference (EDD) diagram of CO2 adsorbed over Cr-graphene as an example. There is significant charge accumulation between the O atoms of CO2 and the M atoms, which suggests that the O atoms are polarized by the positively charged M atoms on the surface. It can be attributed to the more favorable interaction between the lone pair of electrons on the O atom of CO2 and the outer orbitals of the metal atoms, where the d orbitals of the metal hybridize with the p orbitals of the O atom, resulting in a more stable adsorption conformation [33]. The two adsorption configurations align with those reported by Baei et al. [34] (vertical adsorption) and Esrafili et al. [15] (carbon–oxygen adsorption), respectively. In addition, the adsorptions of CO2 on double-layer M-graphenes were also explored, and the same stable adsorption configurations mentioned above were also obtained (Fig. S6). The corresponding adsorption energies are higher than those of single-layer graphene, but the difference is not significant and the most stable adsorption configurations remain the same.
3.3 Hydrogenation mechanism of CO2 on the catalyst surface
The competitive pathway diagram for the hydrogenation of CO2 to generate the C1 products [26, 35,36,37,38] is displayed in Fig. 4. The initial hydrogenation of CO2 leads to the formation of two key intermediates, i.e., COOH and HCOO. Subsequently, the COOH intermediate undergoes hydrogenation to generate two competing products, i.e. HCOOH and CO. HCOO directly forms HCOOH through hydrogenation. Then, HCOOH continues to be hydrogenated and dehydrated to yield CH3OH and CH4, involving the key intermediates HCO and HCOH. In the parallel pathway, the hydrogenation of CO can also produce CH3OH and CH4 involving the same key intermediates (HCO and HCOH). Based on the competitive pathway diagram, the mechanisms of CO2 hydrogenation to C1 products are revealed by discussing each step of the hydrogenation reaction (Reactions 1–5).
3.3.1 The first hydrogenation step
Both the linear vertical adsorption configuration and the bent carbon–oxygen co-adsorption configuration of CO2 (Fig. 3) are considered to study the first hydrogenation step [CO2 + H]* → COOH*/HCOO* (Reaction 1) over the six M-graphenes. Based on these two configurations, intermediate COOH can be formed through Reactions 1–1 and 1–1', and HCOO formation occurs via the competing Reactions 1–2 and 1–2'. The energy profiles and corresponding optimized geometries of these hydrogenation reactions are illustrated in Fig. 5.
During Reaction 1–1, the vertical adsorbed CO2 on the M site and the surrounding adsorbed H on the C site (IS1) transform into intermediate COOH (IM1-1) through the formation of a new O − H bond. According to TS1 shown in Fig. 5, CO2 bends and bonds with the adsorbed H atom through the O atom that far from the catalyst surface. In the IM1-1 state, COOH adsorbs over the M site through both the O and C atoms (V, Cr) or only the C atom (Mn, Ni, Mo, Ta). The processes of IS1 → IM1-1 are exothermic reactions (△E = -81.60 − -127.59 kJ/mol) and the energy barriers range from 166.82 to 214.61 kJ/mol for the six M-graphenes. Among them, COOH is easily formed on V-graphene (166.82 kJ/mol), while it is difficult to be obtained on Ta-graphene (214.61 kJ/mol). As for Reaction 1–2, the HCOO intermediate (IM1-2) is generated via the formation of the C − H bond. Although the angle change of O − C − O during the transition state (TS2) is very small, it keeps decreasing during the whole hydrogenation step. As a result, HCOO adsorbs over the M site through its two O sites except over the Ni site in the final IM1-2 state. The processes of IS1 → IM1-2 are also exothermic (△E = -94.58 − -262.42 kJ/mol) and the activation energies are in the range of 137.49 − 189.31 kJ/mol. The HCOO formation on Ni-graphene has a manifest advantage compared with other M-graphenes due to the quite low energy barrier (Ebarrier = 137.49 kJ/mol). As for the same M-graphene, the formation of HCOO is generally more favorable than COOH from the vertical adsorption of CO2 (Table S6). Particularly, the HCOO formation over Ni-graphene has an obvious advantage due to the lowest energy barrier.
In Reactions 1–1' and 1–2', the hydrogenation initiates from a different CO2 adsorption configuration (carbon–oxygen co-adsorption, IS2). The formation processes of COOH (IM1-1) and HCOO (IM1-2) are similar to Reactions 1–1 and 1–2, with identical adsorption states (COOH* and HCOO*). For IS2 → IM1-1, the energy barriers are 177.16 − 227.78 kJ/mol with the reaction energies range of -49.12 − -100.83 kJ/mol. Similarly, the IS2 → IM1-2 processes are also exothermic (△E = -159.34 − -213.96 kJ/mol) and activation energies are in the range of 137.49 − 189.31 kJ/mol. Upon comparing the energy barrier values for COOH and HCOO formation over the same M-graphene (Table S4), it is observed that the V- and Cr-graphene catalyst surfaces are beneficial for the production of COOH, whereas the Mn-, Ni-, Mo- and Ta-graphene catalyst surfaces are more favorable for the generation of HCOO. Regarding the hydrogenation energy barriers. In total, in spite of the initial CO2 adsorption configuration, the formation of HCOO is more conductive than COOH. The type of active M atom demonstrates selectivity towards hydrogenation intermediates by influencing the initial adsorption and transition state energy.
3.3.2 The second hydrogenation step
According to Fig. 4, COOH undergoes further hydrogenation via Reactions 2–1, 2–2, and 2–3, leading to the formation of C(OH)2, CO, and HCOOH, respectively. HCOO can also convert into HCOOH through Reaction 2–4, and it may be also hydrogenated into H2COO via Reaction 2–5. Figure 6 presents the energy profiles and the optimized geometries of the five hydrogenation reactions.
In Reaction 2–1, the COOH intermediate is adsorbed on V- and Cr-graphene with the M atom interacting via both C and O atoms of COOH in the IM2 state. Along with the formation of a new O − H bond, the generated C(OH)2 intermediate can only interact with the M atom solely via the C atom on the V- and Cr-graphene. Differently, both COOH and C(OH)2 are anchored only by the C atom over other catalysts. Reaction 2–2 is a deoxygenation step in the form of H2O, the distance between C and hydroxyl O of COOH continuously expands as the adsorbed H approaches the hydroxyl O atom, ultimately producing CO and H2O in the final IM2-2 state. Reaction 2–3 results in the conversion from COOH to HCOOH by forming a new C − H bond. Correspondingly, it is the O atom that interacts with the M site in the final IM2-3 state. As indicated in Fig. 6, the reactions of [COOH + H]* → C(OH)2*/[CO* + H2O]/HCOOH* over the M-graphenes are predominantly exothermic, except [COOH + H]* → C(OH)2* on Ta-graphene, which is endothermic (10.07 kJ/mol). The corresponding activation barriers are 200.10/47.90/151.87 kJ/mol (V-graphene), 190.49/178.05/267.78 kJ/mol (Cr-graphene), 161.26/220.99/184.28 kJ/mol (Mn-graphene), 132.95/92.04/127.19 kJ/mol (Ni-graphene), 171.28/32.02/165.64 kJ/mol (Mo-graphene), and 213.60/178.33/153.62 kJ/mol (Ta-graphene) (Table S7). The hydrogenation of COOH on the surface of V-, Cr-, Ni- and Mo-graphene catalysts is more conducive to the production of CO. For HCOOH and C(OH)2, they are favorable to be formed on Ta-graphene and V-graphene, respectively. It is worth noting that Mo-graphene has a unique advantage in producing CO due to the remarkably low energy barrier of 32.02 kJ/mol. This conclusion is consistent with the phenomenon that the C − O bond of COOH in the transition state (TS4) has been fractured, which has not been observed on other catalysts (Fig. 6(b)). Furthermore, Ni-graphene is the most effective for forming C(OH)2 and HCOOH (Ebarrier = 132.95 and 127.19 and kJ/mol).
In Reaction 2–4, HCOO is adsorbed on the M site of the catalyst in the IM2' state through two O atoms. The combination of oxygen and hydrogen atoms leads to the decrease of the O − C − H angle, resulting in the formation of HCOOH. In the IM2-4 state, HCOOH remains adsorbed at the M site by two oxygen atoms. For Reaction 2–5, HCOO transforms into H2COO via C − H bonding, and the corresponding double O co-adsorption remains constant. Taking V-graphene as an example, the distances between H and C atoms gradually increase (3.644 Å → 2.489 Å → 1.106 Å), and a similar trend is observed in other catalysts. As shown in Fig. 6 and Table S5, the comparison of energy barriers reveals that HCOO adsorbed on the surface of Cr-, Mn-, Ni- and Mo-graphene catalysts facilitates the hydrogenation of the O atom to form the O − H bonding, with the formation of HCOOH. Ni-graphene exhibits the best selectivity for HCOOH generation (Ebarrier = 99.30 kJ/mol). Over the V- and Ta-graphene surfaces, the dominant reaction is [HCOO + H]* → H2COO* (Ebarrier = 174.74 and 171.59 kJ/mol).
3.3.3 The third hydrogenation step
To reveal the catalytic selectivity for the intermediate products CO and HCOOH, the subsequent hydrogenation of the intermediate products is very important. Following Fig. 4, four hydrogenation reactions are further studied in the third hydrogenation step, i.e., [C(OH)2 + H]* → [COH + H2O]*, [CO + H]* → HCO*, [HCOOH + H]* → HCO* + H2O and [H2COO + H]* → H2COOH*. The energy profiles and optimized structures of these hydrogenation reactions are shown in Fig. 7.
The hydrogenation process of C(OH)2 in Reaction 3–1 involves the combination of an adsorbed hydrogen atom and a hydroxyl oxygen atom of C(OH)2. This results in the formation of a new O − H bond while simultaneously breaking the C − O bond. Consequently, this process produces both CO and H2O in the final IM 3–1 state. The reaction energies of [C(OH)2 + H]* → [COH + H2O]* on the catalysts range between -5.86 and 67.00 kJ/mol, with activation energies of 96.16 − 251.11 kJ/mol. In Reaction 3–2, CO is adsorbed on the M-site of the catalyst through the C atom in the initial IM3' state. The C and H atoms bond together and the angle of O − C − H decreases continuously to form HCO. In the final IM3-2 state, HCO adsorps at the M (Mn, Ni) site only through its C atom, whereas both C and O atoms are adsorbed at the M site over V-, Cr-, Mo-, and Ta-graphene. The reactions of [CO + H]* → HCO* are endothermic on the Mn-, Ni-, and Mo-graphene and exothermic on the Cr-, V-, and Ta-graphene, featuring energy barriers of 19.62 − 100.46 kJ/mol (Table S8). Notably, a competing reaction involves CO potentially fracturing to form carbon deposition. The reaction of CO* → [C + O]* is depicted in Fig. S7, and the activation energies are in the range of 427.63 − 486.99 kJ/mol. The high energy barriers indicate a low likelihood of carbon deposition through CO fracture. Reaction 3–3 is also a dehydration process via hydrogenation. The hydroxyl O atom of the adsorbed HCOH is attacked by the adsorbed H atom, with another O atom of HCOH interacting with the M site. A new O − H bond is generated, accompanied by the cracking of the C − O bond. As a result, HCO and H2O are generated in the final IM3-2 state. The reactions of [HCOOH + H]* → [HCO + H2O]* are endothermic on Cr-, Mn-, and V-graphene and exothermic on Ni-, Mo-, and Ta-graphene. The activation energies are in the range of 87.95 − 182.69 kJ/mol and the reaction on V-graphene is the most advantageous (Ebarrier = 87.95 kJ/mol). In Reaction 3–4, H2COO initially adsorbs on the M-site of the catalyst through double oxygen atoms in the IM3''' state. One of the O atoms of H2COO bonds with the H atom, causing the continuous decrease of the O − C − H angle. In the final IM3-4 state, H2COOH is generated and adsorbs at the M site (Mn, Ni, Mo) through individual oxygen atoms, while double oxygen atoms adsorb at the M site on V-, Cr-, and Ta-graphene. The processes are exothermic with energy barriers ranging from 65.14 to 123.61 kJ/mol (Table S6).
3.3.4 The fourth hydrogenation step
As for the fourth hydrogenation step (Fig. 4), COH is further hydrogenated to form HCOH (Reaction 4–1), HCO can be converted into HCOH and H2CO through competing Reactions 4–2 and 4–3, H2COOH is further hydrogenated to form H2CO (Reaction 4–4). The detailed hydrogenation processes are discussed below with the corresponding optimized structures and energy profiles represented in Fig. 8.
In reaction 4–1, COH initially adsorbs at the M site of the catalyst through the C atom. As the reaction progresses, COH tilts away from the M site, and the adsorbed H atom approaches the C atom, resulting in the formation of HCOH (IM4-1) through the creation of a new C − H bond. The processes of [COH + H]* → HCOH* are exothermic reactions in the range of -114.55 − -147.98 kJ/mol. Table S9 shows that the corresponding energy barrier values are relatively low (15.37 to 55.43 kJ/mol), indicating that the hydrogenation reaction occurs readily on the catalysts, with the most favorable one on Mn-graphene (15.37 kJ/mol).
For Reaction 4–2, HCO is also adsorbed at the M site of the catalyst through the C atom. The process and product are similar to Reaction 4–1. The difference is that HCOH is generated through O − H bonding in Reaction 4–2. For instance, on V-graphene, the distances between H and O atoms decrease progressively (2.780 Å → 1.950 Å → 0.979 Å), and the trends are observed with other catalysts. Reaction 4–3, while starting with the same adsorption state as Reaction 4–2, results in different products and involves distinct transition states. Here, HCO generates H2CO through the formation of a new C − H bond. During this process, HCO moves downward continuously, and the adsorption via the C atom of HCO on the M site is transformed into the adsorption via the O atom of H2CO. As shown in Fig. 8 and Table S9, the reactions of [HCO + H]* → HCOH*/H2CO* are exothermic with activation energies of 226.91/140.94 kJ/mol (V-graphene), 216.86/164.84 kJ/mol (Cr-graphene), 182.41/180.24 kJ/mol (Mn-graphene), 177.75/147.31 kJ/mol (Ni-graphene), 179.60/31.50 kJ/mol (Mo-graphene), and 208.91/147.79 kJ/mol (Ta-graphene). The energy barrier values indicate that the generation of H2CO has advantages over HCOH on all six catalysts, with Mo graphene (Ebarrier = 31.50 kJ/mol) being the most efficient.
Reaction 4–4 is a dehydration process through hydrogenation. H2COOH is adsorbed at the M site via a single oxygen atom on Cr-, Mn-, and Mo-graphene and by double oxygen atoms on V-, Ni-, and Ta-graphene. The reaction process produces a new O − H bond, accompanied by the fracture of the C − O bond. As a result, H2CO and H2O are generated in the final IM4-4 state. The energy barriers of [H2COOH + H]* → [H2CO + H2O]* range from 58.96 to 210.39 kJ/mol (Table S9), and the reaction on Ta-graphene is the most favorable (Ebarrier = 58.96 kJ/mol).
3.3.5 The fifth hydrogenation step
As illustrated in Fig. 4, H2COH and HC are generated from the hydrogenation of HCOH via Reactions 5–1 and 5–2, and H2COH and H3CO are obtained from H2CO via Reactions 5–3 and 5–4. The energy profiles and optimized structures of these reactions are displayed in Fig. 9.
In reaction 5–1, HCOH is adsorbed on the M site of the catalyst through C atoms in the initial IM5 state. The adsorbed H atom attacks the C atom of HCOH, and the angle of O − C − H decreases continuously to form H2COH. In the final IM5-1 state, H2COH is adsorbed at the M site by C and O atoms. In the case of Reaction 5–2, the distance between C and O of HCOH continues to expand when the adsorbed H atom attacks the hydroxyl O atom, ultimately leading to the formation of HC and H2O (IM5-2). As shown in Fig. 9 and Table S10, the reactions of [HCOH + H]* → H2COH*/[HC* + H2O] are thermodynamically downhill, and corresponding activation energies are 48.83/165.82 kJ/mol (V-graphene), 45.09/174.85 kJ/mol (Cr-graphene), 60.25/176.06 kJ/mol (Mn-graphene), 74.66/151.19 kJ/mol (Ni-graphene), 117.77/232.14 kJ/mol (Mo-graphene), and 113.79/162.76 kJ/mol (Ta-graphene). The energy barriers for H2COH formation are consistently lower than those for HC across all catalysts, signifying that the [HCOH + H]* → H2COH* reaction predominantly occurs on six types of graphene catalysts, with Cr-graphene being the most advantage (45.09 kJ/mol). In addition, the hydrogenation of C to HC is further analyzed, and the optimized structure and energy profile are represented in Fig. S8. The energy barriers for the reaction [C + H]* → HC* are 17.98 − 40.12 kJ/mol, which indicates that C is easily hydrogenated and the catalyst surfaces are not susceptible to carbon deposition.
Reaction 5–3, akin to Reaction 5–1, also leads to the formation of H2COH. In the initial IM5’ state, H2CO interacts with the M atom through its O atom, which is attacked by the adsorbed H atom (TS18). Concurrently, the C − O linkage of the intermediate continuously tilts towards the plane of the catalysts, leading to the co-adsorption of the C and O atoms of H2COH at the M site in the IM5-1 state. As for Reaction 5–4, the adsorbed H atom attacks the C atom of H2CO. Taking V-graphene for example, the distance of the H atom between the C atom of H2CO decreases continuously on V-graphene (2.990 Å → 2.312 Å → 1.103 Å), and the distance of the resulting H3CO from the V site also decreases (1.967 Å → 1.829 Å). This pattern is consistent across various catalysts. The reaction energies of [H2CO + H]* → H2COH*/H3CO* range from -28.76 to 72.66 and -141.17 to -50.35 kJ/mol, respectively. The comparison of energy barriers (Table S10) reveals that H2CO adsorbed on the surface of V-, Cr-, and Ta-graphene catalysts is feasible to form H2COH and Ta-graphene shows the best activity (Ebarrier = 70.12 kJ/mol). Meanwhile, H2CO adsorbed on the surfaces of Mn-, Ni-, and Mo-graphene catalysts is conducive to H3CO generation, with H3CO being the most dominant on Mo graphene (Ebarrier = 39.17 kJ/mol). As shown in Fig. 4, the generated H2COH, H3CO, and CH are the precursors of CH3OH and CH4 respectively. The selectivity of the catalysts for CH3OH and CH4 will be discussed below.
3.4 Discussion on the selectivity of C1 products over different M-graphenes
Based on the above calculation results, the selectivity of C1 products (CO, HCOOH, CH4, CH3OH) from CO2 over the six M-graphene catalysts is carefully discussed below, and the corresponding pathways are csummarized in Fig. 10.
3.4.1 Selectivity in the generation and conversion of CO
The formation path of CO is unique, involving two-step hydrogenation, i.e., CO2* → COOH* → CO*. Based on Reaction 1, adsorption configurations of CO2 (carbon–oxygen co-adsorption) on V- and Cr-graphene are beneficial to the formation of COOH. The absorbed COOH and H form CO and H2O via O − H bond synthesis and O − O bond fracture, which is still favorable on the surface of V- and Cr-graphene. Meanwhile, it can be seen from Reactions 2 that COOH is also conducive to the formation of CO on Ni- and Mo-graphene. Subsequently, CO is easily hydrogenated into HCO on these four catalysts, with the corresponding energy barriers ranging from 19.62 to 52.53 kJ/mol. Meanwhile, the adsorption energy of CO on the catalyst surface ranges from -396.90 to -1067.24 kJ/mol (Fig. S9), which belongs to chemisorption. This indicates that the product is not susceptible to spontaneous detachment from the catalyst surface. It implies the selective production of CO can be unfulfilled over V- and Cr-graphene unless CO can be removed from the reaction zone to avoid further hydrogenation.
3.4.2 Selectivity in the generation and conversion of HCOOH
There are two pathways for the formation of HCOOH by a two-step hydrogenation, i.e., CO2* → COOH*/HCOO* → HCOOH*. Based on the fact that both COOH and HCOO can generate HCOOH, the second step hydrogenation of CO2 is the key to the selective production of HCOOH. According to Reactions 2, COOH exhibits a tendency to undergo hydrogenation, resulting in the formation of new C − H bonds, thereby generating HCOOH on the surface of Ta-graphene (Ebarrier = 153.62 kJ/mol). Subsequently, HCOOH is further hydrogenated to form HCO with an activation energy of 149.02 kJ/mol. It is observed that the activation energy for the generation of HCOOH is greater than that of continuous hydrogenation, indicating that HCOOH can be further hydrogenated on Ta-graphene.
For HCOO, it tends to undergo hydrogenation to form new O − H bonds to generate HCOOH on Cr-, Mn-, Ni- and Mo-graphene surfaces, and the corresponding activation barriers are 172.59, 164.27, 99.30, and 167.33 kJ/mol, respectively. The HCOOH adsorbed on Mn- and Mo-graphene continues to hydrogenate to generate HCO, with the corresponding activation energies of 145.03 and 147.00 kJ/mol, respectively, which are lower than the activation energies for generating HCOOH. As for Cr- and Ni-graphene, HCOOH is not prone to subsequent hydrogenation, since the activation energies for HCOOH hydrogenation to generate HCO (182.69 and 150.10 kJ/mol) are higher than those for HCOOH generation. It suggests that the further hydrogenation of HCOOH over Cr- and Ni-graphene catalysts is less feasible.
In summary, Cr- and Ni-graphene show the potential to achieve the selective production of HCOOH. As depicted in Fig. 10(a), the whole reaction process can be expressed by the general reaction equation: CO2* → HCOO* → HCOOH*. Among them, the rate-determining step (RDS) on Cr-graphene is HCOO* → HCOOH* (172.59 kJ/mol), and the RDS on Ni-graphene is CO2* → HCOO* (137.49 kJ/mol). It is in accordance with the research of Chen et al. [39] on the selective generation of HCOOH by anchoring Cr atoms in C9N4 monolayers for CO2 hydrogenation, and the highly selective hydrogenation of CO2 to HCOOH over Ni catalysts studied by He et al. [40]. In addition, Ni-graphene has more potential than Cr-graphene to catalyze CO2 hydrogenation for the preparation of formic acid in terms of the RDS energy barrier.
3.4.3 Selectivity in the generation of CH4
Two pathways lead to CH4 formation through six and eight steps of hydrogenation, respectively. The corresponding pathways are CO2* → COOH* → C(OH)2* → COH* → HCOH* → HC* → CH4* (route 1) and CO2* → COOH* → CO* → C* → HC* → CH4* (route 2). Since HC is a precursor to CH4, its formation selectivity is key to determining CH4 selectivity. Both pathways involve COOH, according to Reactions 2, COOH is favorable to form CO on V-, Cr-, Ni-, and Mo-graphene, and to form C(OH)2 on Mn-graphene. The energy barriers for the CO* → C* + O* reaction range from 432.86 to 486.99 kJ/mol (Fig. S7), which are all higher than the energy barriers for the CO* → HCO* reaction (Fig. 7). It indicates that CH4 is unlikely to form via route 2 due to the high energy barriers for CO breaking. According to Reactions 5, the energy barriers for the formation of H2COH are significantly lower than those for the formation of HC, and the [HCOH + H]* → H2COH* reaction dominates on the catalyst. It means route 1 is also unviable for CH4 generation. Overall, CO2 is less prone to be hydrogenated into CH4 over the six graphene catalysts.
3.4.4 Selectivity in the generation of CH3OH
CH3OH is generated in multi pathways, as shown in Fig. 4, which involves six steps of hydrogenation reactions. The key intermediate products are CO and HCOOH, and the key intermediates include COOH, HCOO, HCO, HCOH, and H2CO. The formation and continuous hydrogenation of CO and HCOOH have been discussed previously. Based on the intermediate COOH path, the hydrogenation of COOH on the surface of V-, Cr-, Ni- and Mo-graphene catalysts is more conducive to the production of CO. Subsequently, the CO adsorbed on the surface of four catalysts tends to be hydrogenated in two steps to form HCO, H2CO. Then, H2CO adsorbed on the surface of V- and Cr-graphene catalysts is feasible for the formation of H2COH, and it adsorbed on the surface of Ni- and Mo-graphene catalysts tends to generate H3CO. Finally, H2COH and H3CO are hydrogenated to form CH3OH. As for Mn- and Ta-graphene, the hydrogenation of COOH on the surface of Mn- and Ta-graphene catalysts is more conducive to the generation of C(OH)2 and HCOOH, respectively. The C(OH)2 adsorbed on the catalyst tends to be hydrogenated in four steps to produce COH, HCOH, H2COH, and CH3OH, respectively. Similarly, HCOOH adsorbed on the catalyst tends to be hydrogenated in four steps to produce HCO, H2CO, H2COH, and finally CH3OH.
Depending on the intermediate HCOO pathway, the hydrogenation of HCOO on the surface of Cr-, Mn-, Ni-, and Mo-graphene catalysts is more conducive to the production of HCOOH. Based on the selectivity in the formation and conversion of HCOOH, the conversion of HCOOH is challenging on Cr- and Ni-graphene, as it tends not to undergo further hydrogenation. On Mn- and Mo-graphene, the HCOOH progresses through a four-step reaction to produce HCO, H2CO, H3CO and CH3OH, respectively. As for V- and Ta-graphene, the hydrogenation of HCOO on the catalysts is more conducive to the generation of H2COO. The H2COO adsorbed on the catalyst tends to be hydrogenated in four steps to form H2COOH, H2CO, H2COH, and CH3OH, respectively. To sum up, the path of CO2 to CH3OH is shown in Fig. 10. The RDS energy barriers of CO2 to CH3OH for the COOH and HCOO pathways can be compared, i.e., V-graphene (166.82 kJ/mol (CO2* → COOH*, COOH) vs 174.74 kJ/mol (HCOO* → H2COO*, HCOO)), Mn-graphene (192.07 kJ/mol (CO2* → COOH*, COOH) vs 180.68 kJ/mol (CO2* → HCOO*, HCOO)), Mo-graphene (206.83 kJ/mol (CO2* → COOH*, COOH) vs 167.33 kJ/mol (HCOO* → HCOOH*, HCOO)), Ta-graphene (195.71 kJ/mol (CO2* → COOH*, COOH) vs 171.59 kJ/mol (HCOO* → H2COO*, HCOO)). The results show that On V-graphene, the COOH path is favored for methanol generation, while on Mn-, Mo-, and Ta-graphene, the HCOO path is preferred for methanol generation.
In conclusion, graphene catalysts modified with V, Cr, Mn, Ni, Mo, and Ta exhibit selectivity for CH3OH. As for the V-graphene, it aligns with the study of Ling et al. [41] on the efficient conversion of CO2 to CH3OH by loading V atoms on a boron monolayer, which demonstrates the selectivity of the V site for CH3OH. The corresponding reaction pathway is CO2* → COOH* → CO* → HCO* → H2CO* → H2COH* → CH3OH* and the RDS is CO2* → COOH* (166.82 kJ/mol). Regarding Cr-graphene, it is in accordance with Zhang et al.'s research [42] that Mo2C is embedded with transition metal Cr (Cr@Mo2C). Compared with the original Mo2C, CO2 has higher activity and selectivity for CH3OH. The whole pathway is identical to that of V-graphene and the RDS is CO2* → COOH* (183.55 kJ/mol). Concerning Mn-graphene, it is in line with an investigation by Tasfy et al. [43] on the promotion of Mn for the hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts. The reaction pathway is CO2* → HCOO* → HCOOH* → HCO* → H2CO* → H3CO* → CH3OH* and the RDS is CO2* → HCOO* (180.68 kJ/mol). As for the Ni-graphene, it is consistent with the promotion of Ni to CO2 hydrogenation to CH3OH studied by Cannizzaro et al. [44]. The corresponding pathway parallels Cr-graphene except that the intermediate H3CO (H2CO* → H3CO* → CH3OH*) and the RDS is CO2* → COOH* (183.55 kJ/mol). With regard to Mo-graphene, it resonates with the effect of Mo on the promotion of CO2 hydrogenation to CH3OH over Cu/SiO2 catalysts as investigated by Yang et al. [45]. The reaction pathway is similar to Mn-graphene, and the difference lies in the intermediate H2COH, i.e., the path is CO2* → HCOO* → HCOOH* → HCO* → H2CO* → H2COH* → CH3OH* and the RDS is HCOO* → HCOOH* (167.33 kJ/mol). Concerning Ta-graphene, it is consistent with the study of Noh et al. [46] loading Ta atoms on SiO2 for efficient conversion of CO2 to CH3OH. The path is CO2* → HCOO* → H2COO* → H2COOH* → H2CO* → H2COH* → CH3OH* with the RDS being HCOO* → H2COO* (171.59 kJ/mol).
In terms of the catalytic capacity of six M-graphenes for CO2 hydrogenation to methanol, the corresponding RDS energy barriers are 166.82 kJ/mol (V-graphene), 183.55 kJ/mol (Cr-graphene), 180.68 kJ/mol (Mn-graphene), 183.55 kJ/mol (Ni-graphene), 167.33 kJ/mol (Mo-graphene), and 171.59 kJ/mol (Ta-graphene), respectively. This indicates that V-graphene and Mo-graphene are more favorable to catalyze the hydrogenation process compared to other catalysts. Notably, the catalyst model and reaction environment in this article are based on idealized settings, which can provide certain guidance for the preparation, screening, and industrial application of catalysts. However, in practical applications, it is also necessary to consider the effects of reaction conditions such as preparation process, temperature, and pressure on catalyst performance.
4 Conclusions
In this study, the potential of metal-doped graphene catalysts in the hydrogenation of CO2 into C1 products was investigated based on DFT calculations. The hydrogenation mechanism and product selectivity for various C1 compounds are carefully discussed.
Firstly, 21 different M-graphene catalysts were screened in combination with the dissociation ability of hydrogen over the catalysts and the energy band gaps of the catalysts to obtain six candidate catalysts, i.e., V, Cr, Mn, Ni, Mo, and Ta-graphene. The electronic structures indicate the six candidate metals have obvious charge transfer with C, which is beneficial for attracting and activating molecules.
Subsequently, further analysis detailed the CO2 adsorption configurations on these catalyst surfaces, including vertical adsorption and carbon–oxygen co-adsorption. The adsorption energy and EDD indicate that both types of adsorption are stable adsorption configurations.
Finally, based on the adsorption configuration of CO2, the competitive hydrogenation reaction of CO2 was explored. The CO2 hydrogenation mechanisms elucidate the preferential formation of C1 products on the catalyst surface, i.e., Cr- and Ni-graphene favor the production of HCOOH and CH3OH, whereas V-, Mn-, Mo-, and Ta-graphene primarily yield CH3OH.
Availability of data and materials
The data and materials are available upon reasonable request.
Abbreviations
- DFT:
-
Density functional theory
- PBE:
-
Perdew-Burke-Ernzerhof
- DNP:
-
Double numerical basis
- E ads :
-
Adsorption energy
- E barrier :
-
Energy barrier
- E d :
-
Doping energy
- E Diss_barrier :
-
Dissociation energy barrier
- E gap :
-
Energy band gap
- EDD:
-
Electron density difference
- RDS:
-
Rate-determining step
- TS:
-
Transition state
- IM:
-
Intermediate
References
Zhang FZ, Xu RN, He YF, Fang X, Jiang PX (2022) Analysis and optimization of energy flow in the full chain of carbon dioxide capture and oil recovery. Carbon Neutrality 1(1):31
Zhao C, Ju S, Xue Y, Ren T, Ji Y, Chen X (2022) China’s energy transitions for carbon neutrality: challenges and opportunities. Carbon Neutrality 1(1):7
Chen C, Jiao F, Lu B, Liu T, Liu Q, Jin H (2023) Challenges and perspectives for solar fuel production from water/carbon dioxide with thermochemical cycles. Carbon Neutrality 2(1):9
Zhou C, Ge Z, Wang Y, Shang F, Guo L (2023) Experimental study on supercritical carbon dioxide gasification of biomass. Carbon Neutrality 2(1):2
Wang Y, He D, Chen H, Wang D (2019) Catalysts in electro-, photo- and photoelectrocatalytic CO2 reduction reactions. J Photochem Photobiol, C 40:117–149
Jiang X, Wang X, Nie X, Koizumi N, Guo X, Song C (2018) CO2 hydrogenation to methanol on Pd-Cu bimetallic catalysts: H2/CO2 ratio dependence and surface species. Catal Today 316:62–70
Novoselov KS, Geim AK, Morozov SV (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: a review of graphene. Chem Rev 110(1):132–145
Gao Q, Li T, Liu C, Sun J, Liu Y, Hou L, Yuan C (2023) Hierarchically porous N-doped carbon framework with enlarged interlayer spacing as dual-carbon electrodes for potassium ion hybrid capacitors. Carbon Neutrality 2(1):18
Li H, Zahra MMA, Bashar BS (2023) A comprehensive investigation of thermal conductivity in of monolayer graphene, helical graphene with different percentages of hydrogen atom: A molecular dynamics approach. Colloids Surf A Physicochem Eng Asp 656:130324
Wang M, Huang M, Luo D (2021) Single-crystal, large-area, fold-free monolayer graphene. Nature 596(7873):519–524
Wei G, Miao YE, Zhang C, Yang Z, Liu Z, Tjiu WW, Liu T (2013) Ni-doped graphene/carbon cryogels and their applications as versatile sorbents for water purification. ACS Appl Mater Interfaces 5(15):7584–7591
Liang X, Ke Q, Zhao X, Chen X (2023) Graphene-supported tin single-atom catalysts for CO2 hydrogenation to HCOOH: A theoretical investigation of performance under different N coordination numbers. ACS Applied Nano Materials 6(6):4489–4498
Tamaki K, Verma P, Yoshii T, Shimojitosho T, Kuwahara Y, Mori K, Yamashita H (2023) Design of Au nanorods-based plasmonic catalyst in combination with nanohybrid Pd-rGO layer for boosting CO2 hydrogenation to formic acid under visible light irradiation. Catal Today 411:113795
Esrafili MD, Dinparast L (2017) A DFT study on the catalytic hydrogenation of CO2 to formic acid over Ti-doped graphene nanoflake. Chem Phys Lett 682:49–54
Ali S, Yasin G, Iqbal R, Huang X, Su J, Ibraheem S, Zhang Z, Wu X, Wahid F, Ismail PM (2022) Porous aza-doped graphene-analogous 2D material a unique catalyst for CO2 conversion to formic-acid by hydrogenation and electroreduction approaches. Molecular Catalysis 524:112285
Zhang R, Huang Z, Li C, Zuo Y, Zhou Y (2019) Monolithic g-C3N4/reduced graphene oxide aerogel with in situ embedding of Pd nanoparticles for hydrogenation of CO2 to CH4. Appl Surf Sci 475:953–960
Xia M, Ding J, Du X, Shang R, Zhong Q (2019) Ambient hydrogenation of CO2 to methane with highly efficient and stable single-atom silver-manganese catalysts. J Alloy Compd 777:406–414
Liu X, Wang Z, Tian Y, Zhao J (2020) Graphdiyne-supported single iron atom: A promising electrocatalyst for carbon dioxide electroreduction into methane and ethanol. J Phys Chem C 124(6):3722–3730
San X, Gong X, Hu Y, Hu Y, Wang G, Qi J, Meng D, Jin Q (2021) Highly dispersed Cu/graphene nanocatalyst guided by MOF structure: Application to methanol synthesis from CO2 hydrogenation. ChemistrySelect 6(24):6115–6118
Ma Q, Geng M, Zhang J, Zhang X, Zhao TS (2019) Enhanced catalytic performance for CO2 hydrogenation to methanol over N-doped graphene incorporated Cu-ZnO-Al2O3 catalysts. ChemistrySelect 4(1):78–83
Wang Y, Ding J, Zhao J, Wang J, Han X, Deng Y, Hu W (2022) Selective electrocatalytic reduction of CO2 to formate via carbon-shell-encapsulated In2O3 nanoparticles/graphene nanohybrids. J Mater Sci Technol 121:220–226
Shi Y, Su W, Kong L, Wang J, Lv P, Hao J, Gao X, Yu G (2022) The homojunction formed by h-In2O3 (110) and c-In2O3 (440) promotes carbon dioxide hydrogenation to methanol on graphene oxide modified In2O3. J Colloid Interface Sci 623:1048–1062
Cheng D, Negreiros FR, Aprà E, Fortunelli A (2013) Computational approaches to the chemical conversion of carbon dioxide. Chemsuschem 6(6):944–965
Yodsin N, Rungnim C, Tungkamani S, Promarak V, Namuangruk S, Jungsuttiwong S (2019) DFT study of catalytic CO2 hydrogenation over Pt-decorated carbon nanocones: H2 dissociation combined with the spillover mechanism. J Phys Chem C 124(3):1941–1949
Su X, Yang X, Zhao B, Huang Y (2017) Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: recent advances and the future directions. J Energy Chem 26(5):854–867
Szkaradek K, Buzar K, Pidko EA, Szyja BM (2018) Supported Ru metalloporphyrins for electrocatalytic CO2 conversion. ChemCatChem 10(8):1814–1820
Chen HZ, Liu J, Li WT, Hu B, Liu XR, Wu YW, Zhang B, Lu Q (2023) Mechanism insights into the decarbonylation of furfural to furan over Ni/MgO: A molecular simulation study. Energy Fuels 37(14):10594–10602
Jiang D, Li H, Cheng X, Wang S, Abomohra A, Cao B (2022) Activation of nitrogen-doped carbon materials on the C-N bond and C–O bond: modeling study toward enhanced pyrolysis products. ACS Sustain Chem Eng 10(23):7473–7484
Zhu D, Zhou Q (2021) Nitrogen doped g-C3N4 with the extremely narrow band gap for excellent photocatalytic activities under visible light. Appl Catal B 281:119474
Chen JH, Ye C, Li YQ (2010) Quantum-mechanical study of effect of lattice defects on surface properties and copper activation of sphalerite surface. TNMSC 20(6):1121–1130
Burghaus U (2014) Surface chemistry of CO2–Adsorption of carbon dioxide on clean surfaces at ultrahigh vacuum. Prog Surf Sci 89(2):161–217
David R, Kabbour H, Pautrat A, Touati N, Whangbo MH, Mentré O (2014) Two-orbital three-electron stabilizing interaction for direct Co2+ As3+ bonds involving square-Planar CoO4 in BaCoAs2O5. Angew Chem 126(12):3175–3178
Baei MT (2013) DFT Study of CO2 adsorption on the Zn12O12 nano-cage. Bull Korean Chem Soc 34(12):3722–3726
Miao B, Ma SK, Wang X, Su H, Chan SH (2016) Catalysis mechanisms of CO2 and CO methanation. Catal Sci Technol 6(12):4048–4058
Peng G, Sibener S, Schatz GC, Mavrikakis M (2012) CO2 hydrogenation to formic acid on Ni (110). Surf Sci 606(13–14):1050–1055
Wu Z, Qin M, Liu Y, Fang HL (2017) Prediction of major impurities during MeOH synthesis over a Cu/ZnO/Al2O3 catalyst. Ind Eng Chem Res 56(49):14430–14436
Qiu M, Tao H, Li R, Li Y, Huang X, Chen W, Su W, Zhang Y (2016) Insight into the mechanism for the methanol synthesis via the hydrogenation of CO2 over a Co-modified Cu (100) surface: A DFT study. J Chem Phys 145(13):134701
Chen JL, Hu HJ, Wei SH (2022) Transition metal anchored on C9N4 as a single-atom catalyst for CO2 hydrogenation: A first-principles study. Chin Phys B 31(10):107306
He CS, Gong L, Zhang J, He PP, Mu Y (2017) Highly selective hydrogenation of CO2 into formic acid on a nano-Ni catalyst at ambient temperature: Process, mechanisms and catalyst stability. J CO2 Util 19:157–164
Ling C, Li Q, Du A, Wang J (2018) Computation-aided design of single-atom catalysts for one-pot CO2 capture, activation, and conversion. ACS Appl Mater Interfaces 10(43):36866–36872
Zhang Y, Cao Z (2021) Tuning the activity of molybdenum carbide MXenes for CO2 electroreduction by embedding the single transition-metal atom. J Phys Chem C 125(24):13331–13342
Tasfy SFH, MohdZabidi NA, Shaharun MS, Subbarao D (2014) Effect of Mn and Pb promoters on the performance of Cu/ZnO-catalyst in CO2 hydrogenation to methanol. Trans Tech Publ, Applied Mechanics and Materials, pp 289–292
Cannizzaro F, Hensen EJ, Filot IA (2023) The Promoting Role of Ni on In2O3 for CO2 Hydrogenation to Methanol. ACS Catal 13:1875–1892
Yang Y, Yao D, Zhang M, Li A, Gao Y, Fayisa BA, Wang MY, Huang S, Wang Y, Ma X (2021) Efficient hydrogenation of CO2-derived ethylene carbonate to methanol and ethylene glycol over Mo-doped Cu/SiO2 catalyst. Catal Today 371:113–119
Noh G, Lam E, Bregante DT, Meyet J, Šot P, Flaherty DW, Copéret C (2021) Lewis acid strength of interfacial metal sites drives CH3OH selectivity and formation rates on Cu-Based CO2 hydrogenation catalysts. Angew Chem 133(17):9736–9745
Acknowledgements
Not applicable.
Funding
The authors thank the National Natural Science Foundation of China (52276189, 52376182, 52106241), Natural Science Foundation of Jiangsu Province (BK20221248), Fundamental Research Funds for the Central Universities (2023JC009), Project of National Center for International Research on Intelligent Nano-Materials and Detection Technology in Environmental Protection, Soochow University (SDGH2201) for financial support.
Author information
Authors and Affiliations
Contributions
HZC: Conceptualization, Investigation, Writing—Original Draft; JL: Conceptualization, Funding acquisition, Writing—Review & Editing; BH: Conceptualization, Funding acquisition, Writing—Review & Editing; XRL: Investigation, Methodology, Writing—Review; HYW: Methodology, Data Validation; JHL: Data Validation, Writing—Review; QL: Funding acquisition, Supervision, Writing—Review & Editing. We ensure that all authors are included in the author list and its order has been agreed by all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Hz., Liu, J., Hu, B. et al. Mechanistic insight into the C1 product selectivity for the catalytic hydrogenation of CO2 over metal-doped graphene. Carb Neutrality 3, 9 (2024). https://doi.org/10.1007/s43979-024-00086-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43979-024-00086-8